

#Pickering emulsion salt water skin
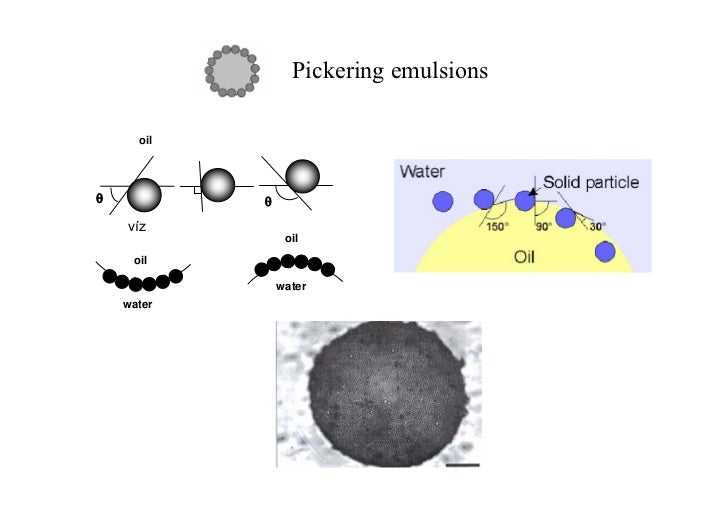
Kinetic studies carried out at varying NaCl concentrations suggested that particle formation in the reaction followed a combination of a coagulative nucleation mechanism, characterized by a clustering process of Janus precursors to form bigger aggregates, and droplet nucleation. Pickering emulsions are increasingly used in the pharmaceutical and cosmetic fields, especially for topical applications, since these systems require solid particles as emulsifiers instead of surfactants which are known to cause skin irritation. 25 mM, fully armored polymeric particles surrounded by a dense layer of adsorbed stabilizing nanogels were formed. In particular, at the highest tested ionic strength, ca. Along with an increase in size, the morphology of these polymer colloids changed from Janus to patchy with an increase in number of nanogels adsorbed on the polymer surface, as a function of the salt concentration in water. This observation challenges current wisdom that so-called Pickering emulsions are at most metastable and points to a new class of mesoscopic equilibrium structures. Via the so-called Pickering mechanism, these colloidal particles can adsorb irreversibly at the oil-water interface, to sterically (i.e. It is shown that an increase in ionic strength of the dispersing medium in these polymerizations led to the formation of latexes of larger diameters. The terms of low sugar, low-fat and low salt are often used in labeling the. We show that under appropriate conditions, mixtures of oil, water, and nanoparticles form thermodynamically stable oil-in-water emulsions with monodisperse droplet diameters in the range of 30-150 nm. NaCl, on the emulsion polymerization is studied. In conventional emulsions, due to their amphipathic properties, stability and emulsification depend on the arrangement of surfactants at the oilwater interface. Nanogels made from crosslinked block copolymer micelles are used as stabilizers in the Pickering emulsion polymerization of styrene. Nanocelluloses are likely to form o/w emulsions, and the emulsions are typically prepared by mechanical treatment of the mixture of oil and nanocellulose aqueous dispersion, in which nanocelluloses are adsorbed at the oil/water interfaces Citation 41 and stabilize the emulsion. We find that particle exchange occurs by two routes: firstly, during a period of unbridging and rebridging whose duration can be tuned by varying the wettability of the particles and secondly.


 0 kommentar(er)
0 kommentar(er)
